EpiGuide Membrane – 50600-03
$144.00 Original price was: $144.00.$139.00Current price is: $139.00.
EpiGuide is a resorbable, synthetic dental membrane that assists in the regeneration of bone and periodontal support tissues.

EpiGuide is a resorbable, synthetic dental membrane that assists in the regeneration of bone and periodontal support tissues.
- High-tech Architecture with Unique 3 Layer Technology
- 100% Synthetic and Completely Resorbable
- Maintains architecture and structural integrity for up to 20 weeks after implantation.
- Complete bioresorption occurs between 6 and 12 months.
- Regenerates tissue despite flap recession or if primary closure is not obtained.
- Compares to GUIDOR ® Bioresorbable Matrix Barrier 1,2
- Available Size: : 18 x 30 mm
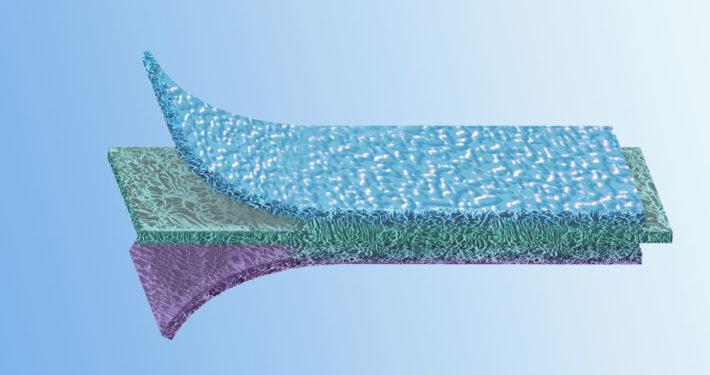
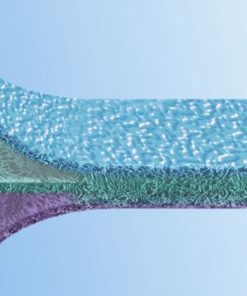
EpiGuide: Unique 3 Layer Technology
Epi-Guide is a uniquely structured three-dimensional bioresorbable membrane with many applications within guided tissue regeneration (GTR) and guided bone regeneration (GBR). This internal structure creates a gradient of density designed to allow fibroblasts and epithelial cells to enter the hollow spaces and to attach themselves to the walls, stabilizing these cells in the process. The innovative structure is easily recognizable in cross-section: The inner layer, featuring large and closed pores, transforms into a chamberlike structure in the intermediate layer and then into the highly porous outer layer, also featuring large pores. The membrane aligns the growth of fibroblasts and epithelial cells so epithelial migration is prevented during subsequent healing stages. The structure and integrity are maintained for more than six weeks after implantation. The resorption of the barrier matrix is completed within six to twelve months

Layer 1: Gingival Interface Numerous voids and intercommunicating pathways enhance fibroblast infiltration and cell attachment.
Layer 2: Inner Surface Inner labyrinth creates pathways, while internal chambers enable collateral circulation and flow of intersititial fluid in the membrane.
Layer 3: Defect Interface Optical porosity supports the uptake of fluid, helps adherence to the tooth surface, and inhibits fibroblast movement.
EpiGuide: Structure and Characteristics
The Epi-Guide® barrier matrix is made from polylactide (PLA) and comes in 18 x 30 mm (0.7 x 1.2 in.) rectangles. For easier differentiation, the surface facing the soft tissue has an embossed relief structure. Epi-Guide® is highly hydrophilic and will accommodate a large amount of blood in the intermediate chamber layer; the ingrowth of epithelial cells is prevented. In this manner, the barrier matrix serves as a placeholder for the development of bone and periodontal tissue.
EpiGuide: Resorption Behavior
It was shown in histological examinations performed six weeks postoperatively that inflammation free collagen fibers had formed in the barrier matrix. The architecture and the structure of the barrier had remained stable. After three weeks, as the formation of collagen fibers continues, bioresorption sets in; the matrix, however, continues to serve its function. After approximately 12 months, Epi-Guide® will have been completely resorbed. No second-stage surgery is required. The membrane surface with its open and interconnected pores counteracts suture dehiscence and gingival recession. It should be emphasized that any dehiscence or recession – should they occur after all – are very well tolerated by the membrane. Regenerates tissue despite flap recession or if primary closure is not obtained.
Epi-Guide in Clinical Studies on Humans
In a multicenter study including 40 patients with bilateral Class II furcations defects, Vernino et al. 1 examined the influence of three-dimensional polylactide barriers (Epi-Guide® and Guidor) on the regeneration of hard tissues. The amount and quality of new bone formed was evaluated one year postoperatively by surgical reentry. The results for three centers showed significantly better results for Epi-Guide® with regard to the reduction of the vertical component. The same study also showed that exposition within the first eight weeks occurred markedly less frequently with EpiGuide® than with the reference product.
EpiGuide Webinar
EpiGuide Case Reports
Cases by: Dr Frederic Hermann, AZZ Ambulantes Zahnmedizinisches Zentrum
Case #1: Periodontological Indication
The patient was 39 years old at baseline. Her general health was good. Extraoral examination did not reveal any pathological findings. The intraoral situation was characterized by soft and hard supragingival concrements. All teeth exhibited positive sensitivity. The gingival margin in all quadrants exhibited only moderate localized inflammatory changes. A swollen and reddened interdental papilla was found between teeth 15 and 16. Probing depths were between 2 and 4 mm, locally reaching 8 mm at 15 and 16. No pathological tooth mobility was present.
The radiological examination showed pronounced radical bone defect reaching the apical third (Figures 2 and 3, pre- and postoperative radiographs).
The microbiological examination of the subgingival plaque demonstrated the presence of Actinobacil lus actinomycetemcomitans and, in connection with the clinical findings, motivated a diagnosis of localized aggressive periodontitis.
Treatment
The surgical therapy was preceded by anti-infectious therapy, consisting of closed subgingival curettage accompanied by antibiotics (amoxicillin + metronidazole; Van Winkelhoff, 1989) and 0.12% chlorhexidine digluconate rinses. When the result of the treatment was assessed 10 weeks later, probing depths of 7 mm persisted at teeth 15 and 16. Subsequent surgical therapy provided for the regeneration of the intraosseous defect using the new resorbable Epi-Guide® barrier matrix (curasan AG, Kleinostheim, Germany) for guided tissue regeneration. Based on the morphology of the defect as uncovered intraoperatively (two-walled defect), additional support was consciously provided by applying a bone regeneration material (B-TCP/Cerasorb® M, 500–1,000 µm, curasan AG) to preserve the cavity. The surgical procedure is illustrated in Figures 4 to 11. The membrane and flap were additionally stabilized by an offset suture with resorbable suture material above the defect area. Vertical interrupted sutures were used to reposition the papilla.
Outcome
The wound healing process was free of complications. An attachment gain of 2.5 mm was subsequently measured. A slight papillary recession of 1 to 3 mm in the regeneration area is frequently seen and could not be predictably avoided even in this case despite the use of microsurgical methods and papilla preservation techniques. The patient should always be fully informed of this risk ahead of the operation, especially in the case of procedures in the esthetic zone. Probing depth decreased to 3 mm, which is a level that the patient can easily maintain, yielding a favorable long-term prognosis of freedom from inflammation if regular recalls are made.
Case #2: Implantological Indication
The patient was 67 years old at baseline. His general health was good. Extraoral examination did not reveal any pathological findings. The alveolar range between teeth 13 and 23 showed moderate vertical and pronounced horizontal atrophy. Following periodontological pretreatment, the existing maxillary telescopic restoration increasingly lost its retention, and it was decided to add abutments by implantological means. In addition, the patient requested a fixed restoration without a palatal bar.
Treatment
Following a minimally invasive incision along the top of the alveolar ridge (13–21) and mobilization of a mucoperiosteal flap, placement of a Revois® implant was accompanied by concurrent widening of the alveolar ridge using the bone-splitting technique. Following placement of the implant, the crestal aspect of the vestibular bony lamella fractured and developed a dehiscence, which was augmented with B-TCP (Cerasorb® M, 500–1,000 µm, curasan AG) and covered with a resorbable membrane (Epi-Guide®, curasan AG; see Figures 12 to18).
Outcome
After tension-free primary wound closure, wound healing proceeded without complications, with no membrane exposure or inflammatory reactions. The vestibular contours of the alveolar ridge could be restored in their entirety.
1. Use of biodegradable polylactic acid barrier materials in the treatment of grade II periodontal furcation defects in humans–Part I: A multicenter investigative clinical study. Int J Periodontics Restorative Dent. 1998 Dec;18(6):572-85. Vernino AR et. al.
2. Guidor is a registered trademark of Sunstar Americas, Inc.
| Weight | 0.2 kg |
|---|---|
| Dimensions | 8 × 8 × 8 cm |
Be the first to review “EpiGuide Membrane – 50600-03”
You must be logged in to post a review.
Related products
Biologics
Biologics
Biologics
































107 reviews for EpiGuide Membrane – 50600-03
There are no reviews yet.