All, The Truth About Titanium Implants
Titanium Corrosion in the Oral Environment
Metals rust and corrode as the result of the metal breaking down when exposed to extreme, wet or acid dominated environments. This is the case of the titanium that is common today.

Titanium is a popular metal that is recognized as more durable and stronger than steel, yet is lighter and more flexible than steel. These properties of titanium make it a popular metal used in chemical plants, airplanes, various military and engineering applications and dentistry. Titanium is also used in rifles and air guns.
Titanium Corrosion Mechanisms in the Oral Environment

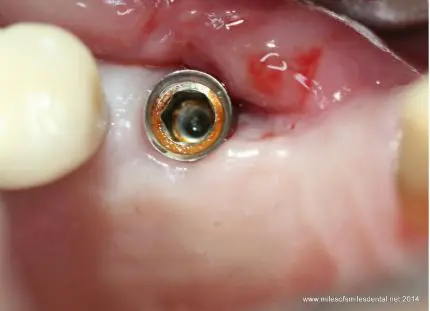
Titanium (Ti) and its alloys are broadly used in the design of dental and orthopedic implants. The corrosion resistance of Ti and its alloys is a result of the material’s ability to spontaneously form passive oxide films (TiO2) when in contact with oxygen. Ti oxide is a stable and dense layer, which acts as a protective barrier to continued metallic oxidation. In the event of damage, TiO2 has the ability to spontaneously reform under normal physiological conditions.
However, events, such as abnormal cyclic loads, implant micromotion, acidic environments and their conjoint effects, can result in permanent breakdown of the oxide film, which may consequently lead to exposure of the bulk metal to an electrolyte.
Corrosion of titanium dental implants has been associated with implant failure and is considered one of the triggering factors for peri-implantitis.
Corrosion of dental implants is concerning, because a large amount of metal ions and debris are generated in this process, of which accumulation may lead to adverse tissue reactions in the oral environment. In summary, the main events linked to Ti implant degradation in the oral environment seem to be related to: (1) electrochemical factors, acidity caused by the presence of inflammatory processes, oral bacteria or the use of solutions that can attack the surface of the implant; (2) mechanical factors, induced by mechanical loads that can lead to fretting and excessive wear of the surface; and (3) synergistic action of electrochemical and mechanical factors (tribocorrosion).
The infiltration of saliva into the multi-metallic structures on titanium implants brings different types of alloys into temporary or permanent contact. In this way a galvanic cell is established as a result of their potential difference. The galvanic cell phenomenon is compounded by another type of corrosion resulting from the geometry of the assembly: localized crevice corrosion.
References:
https://titaniumprocessingcenter.com/does-titanium-rust/
https://www.sciencedirect.com/science/article/pii/0300571294902003
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5452779/



Comments are closed.