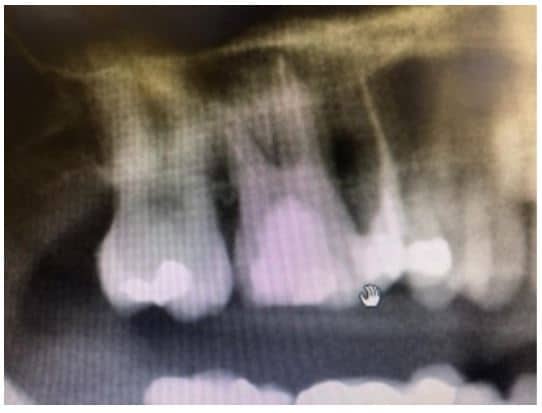
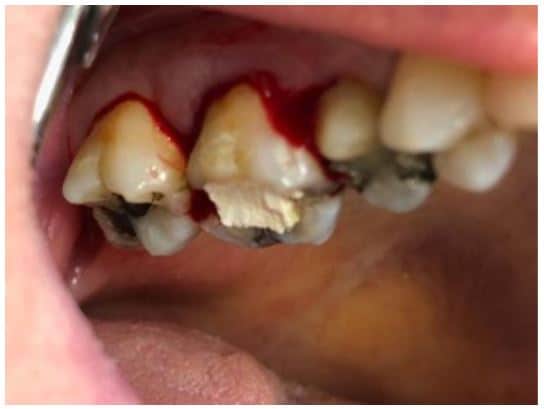
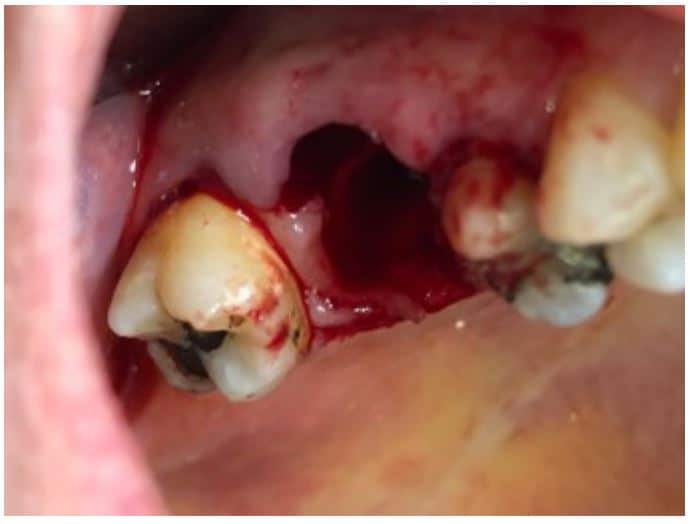
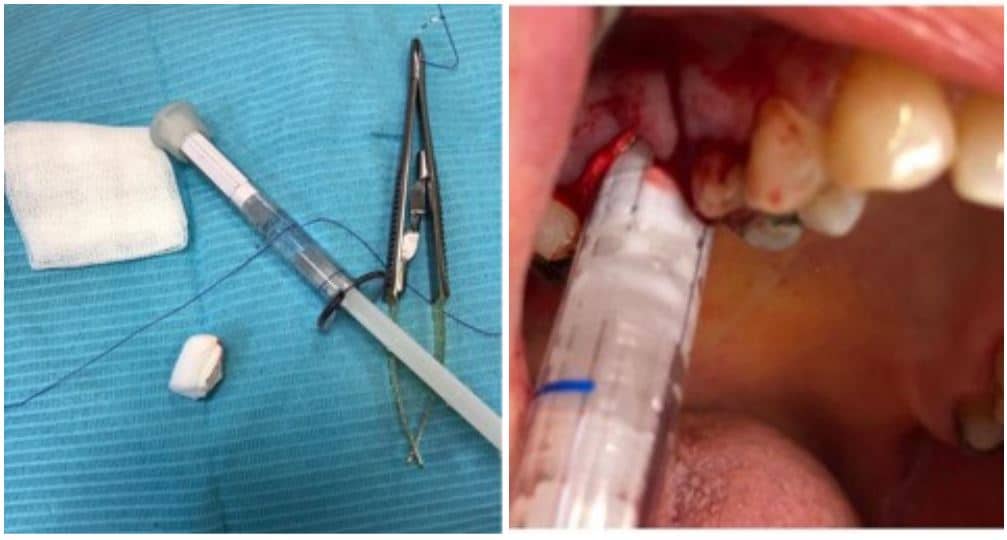
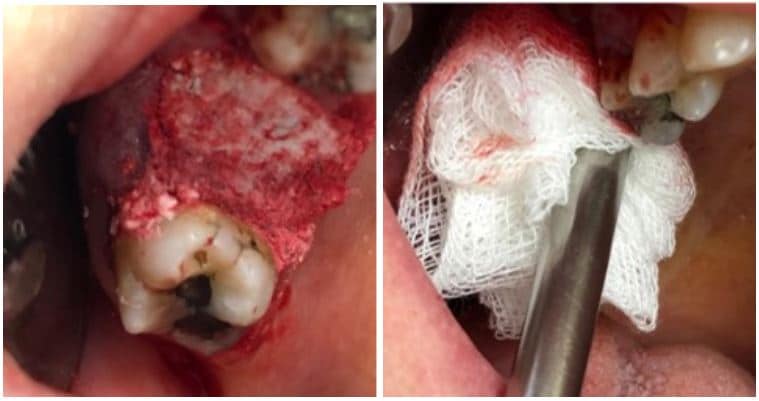
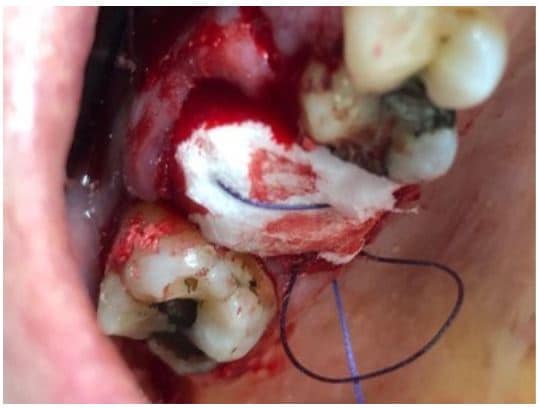
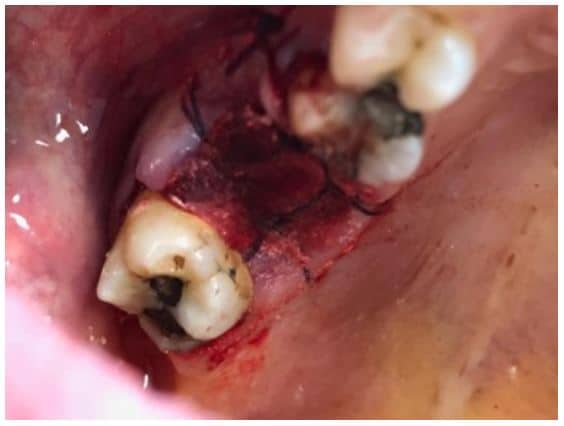
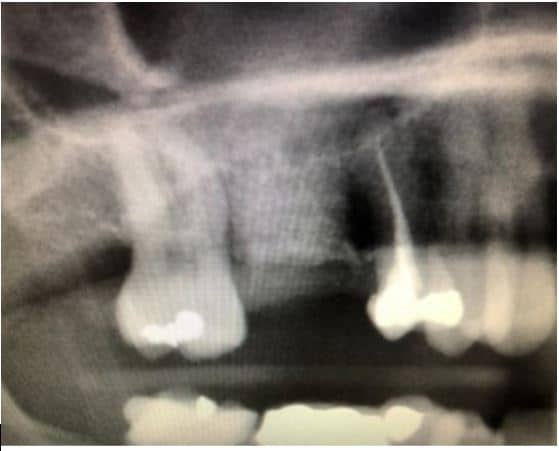
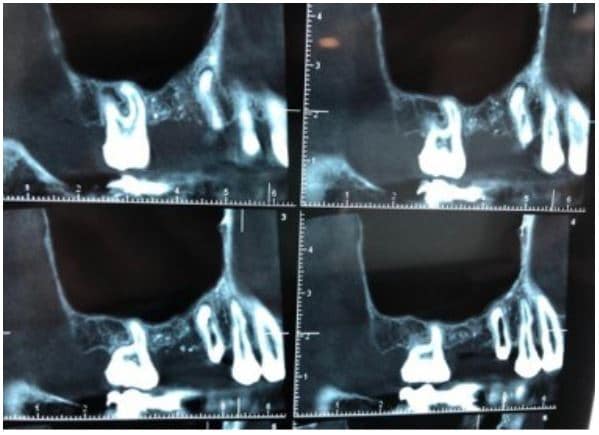
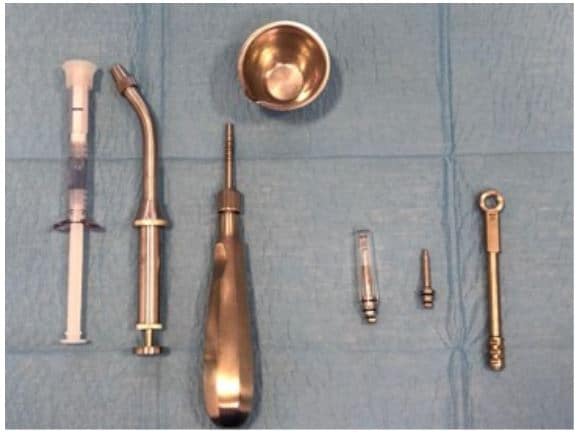
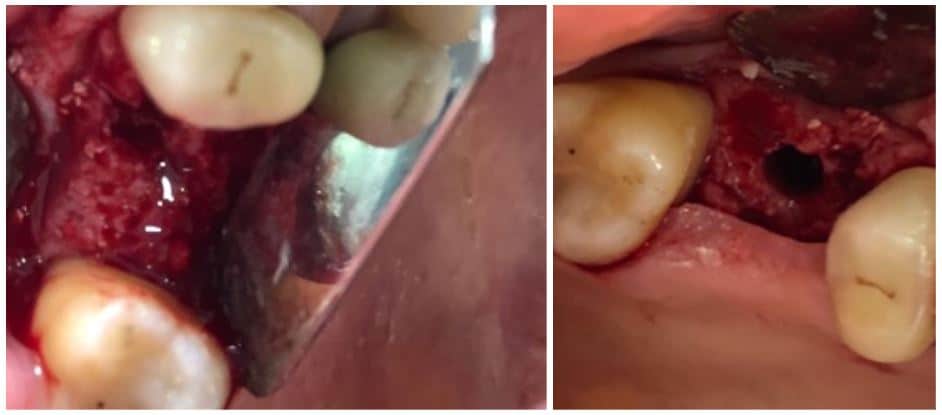
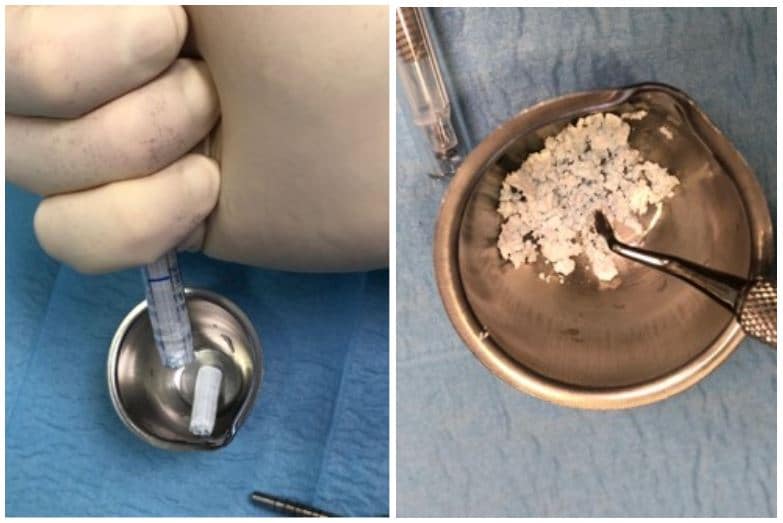
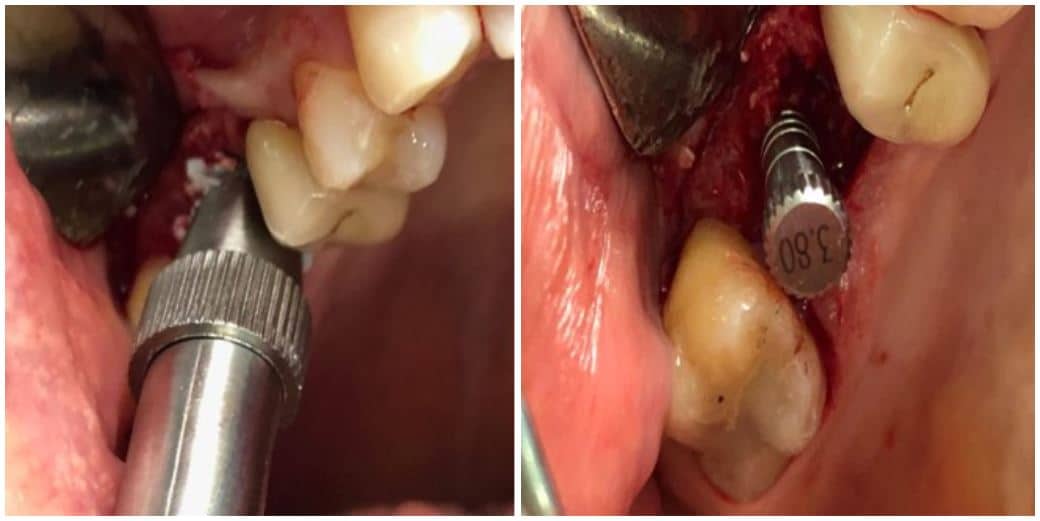
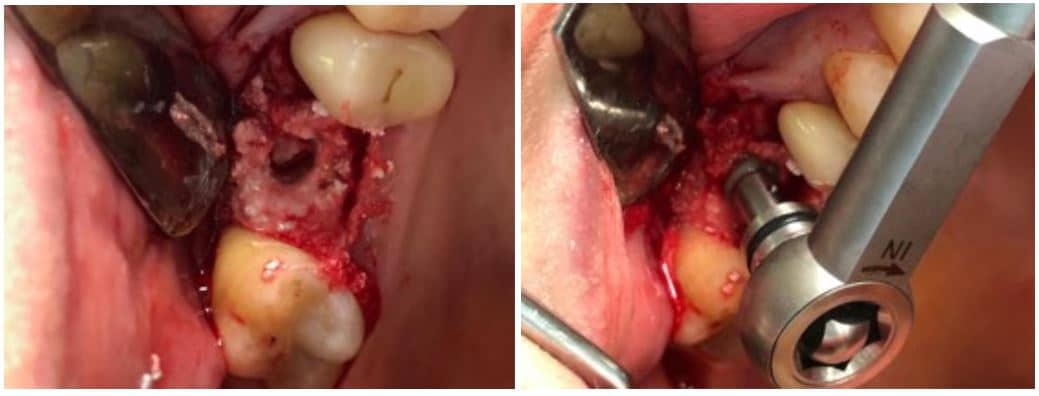
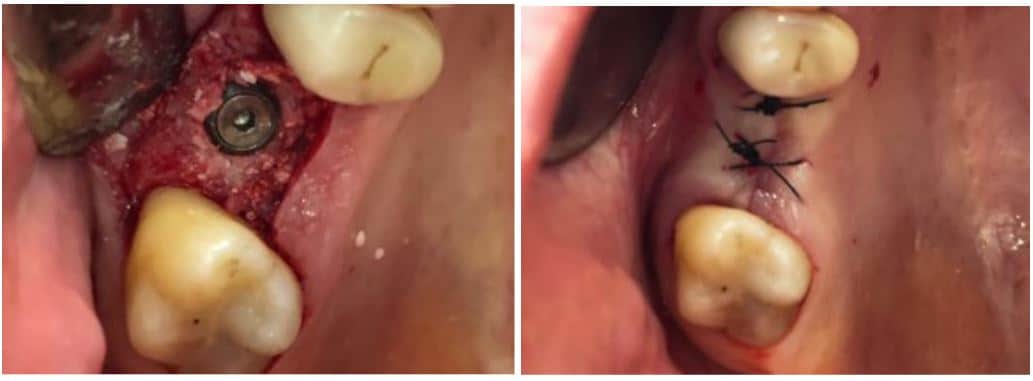
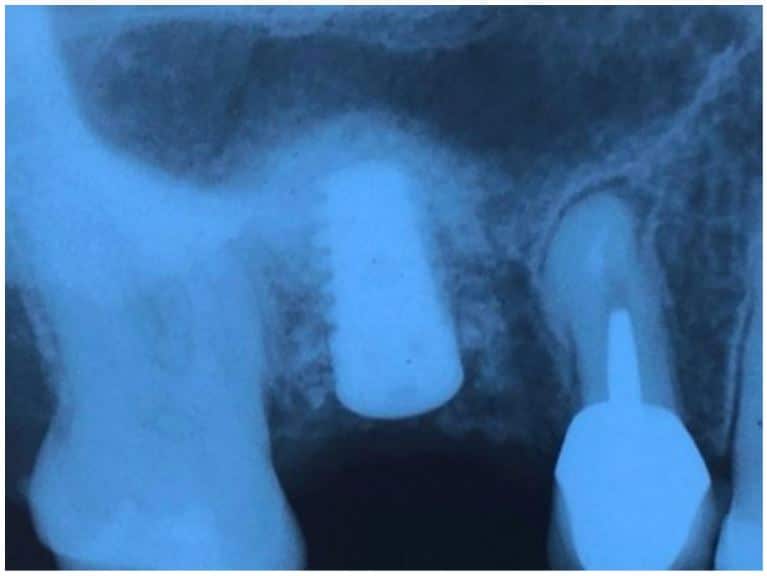
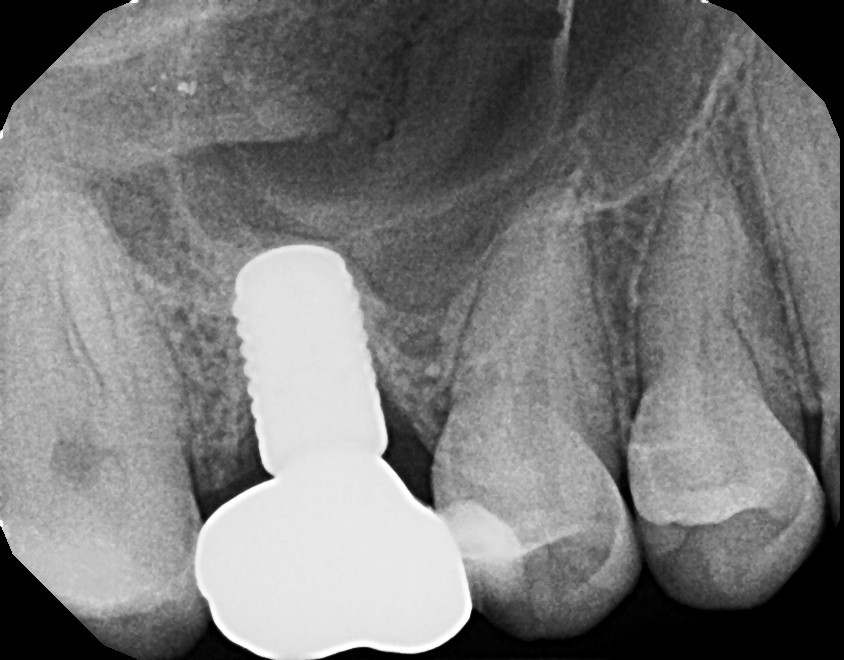
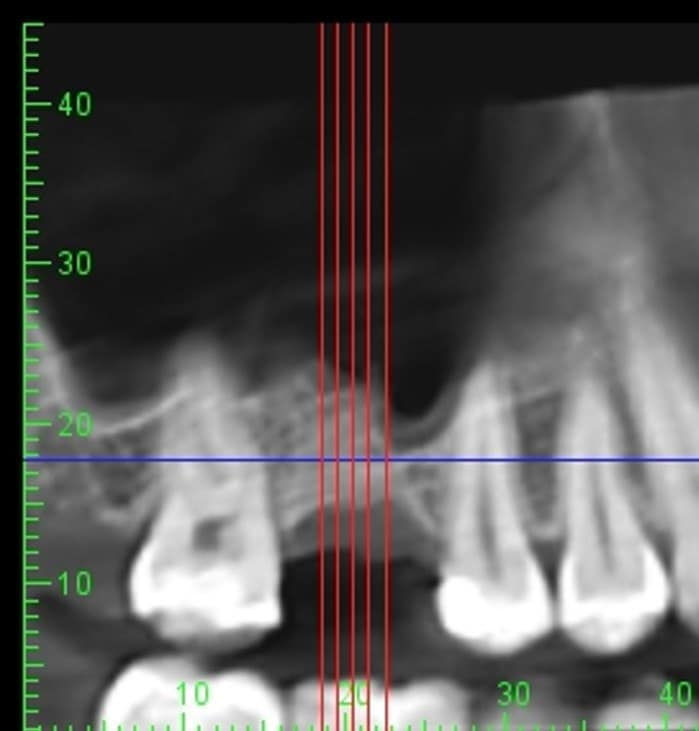
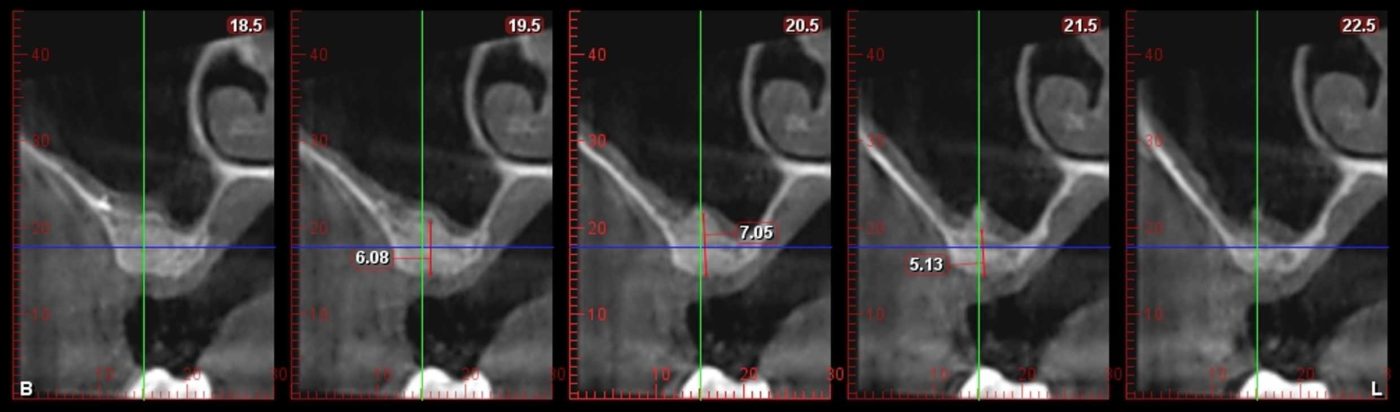
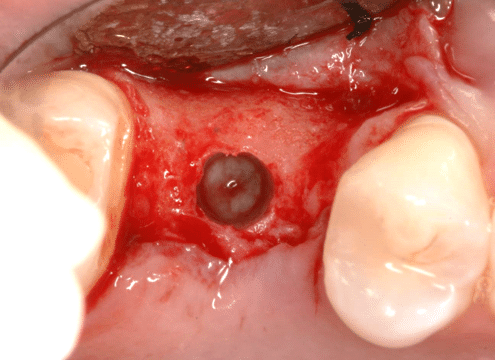
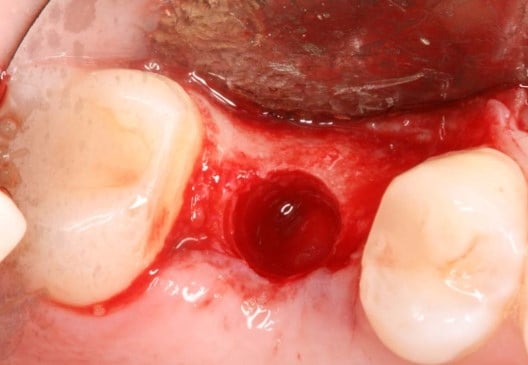
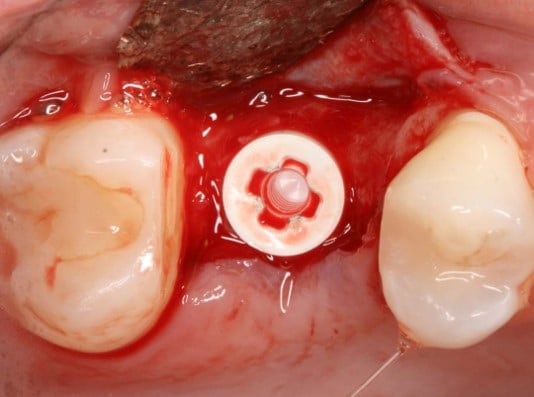
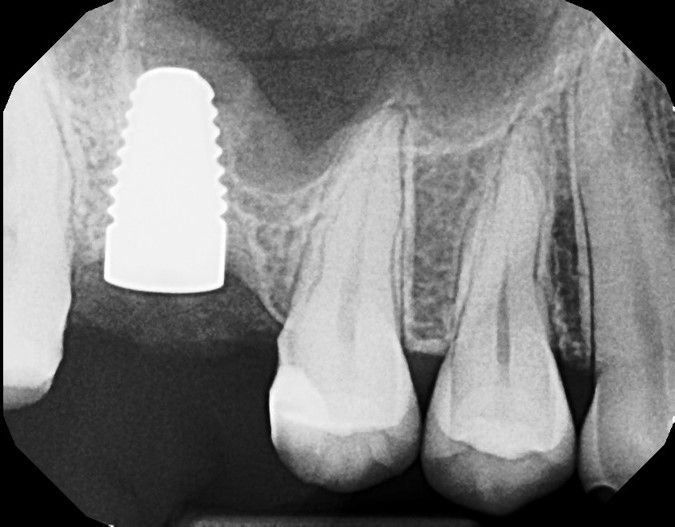
The post Augma Biomaterials Case appeared first on Emerginnova.
]]>The post Augma Biomaterials Case appeared first on Emerginnova.
]]>The post Patient requests removal of a titanium implant and replacement with a ZERAMEX XT implant appeared first on Emerginnova.
]]>The post Patient requests removal of a titanium implant and replacement with a ZERAMEX XT implant appeared first on Emerginnova.
]]>